| Therapies
to Manage Insulin Resistance Improve Response to Interferon-based Therapy in Chronic
Hepatitis C Patients By
Liz Highleyman 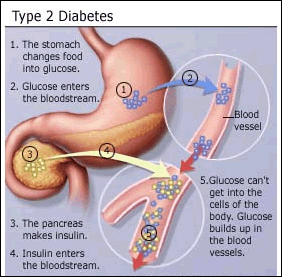 Several
prior studies have shown that people with chronic
hepatitis C virus (HCV) infection are more likely to have insulin
resistance or diabetes, and that these conditions in turn are associated with
poorer response to interferon-based
therapy. This observation has led experts to suggest that managing insulin
resistance might improve hepatitis
C treatment outcomes. Several
prior studies have shown that people with chronic
hepatitis C virus (HCV) infection are more likely to have insulin
resistance or diabetes, and that these conditions in turn are associated with
poorer response to interferon-based
therapy. This observation has led experts to suggest that managing insulin
resistance might improve hepatitis
C treatment outcomes.
Metformin
In
a late-breaker presentation at the recent 59th Annual Meeting
of the American Association for the Study of Liver Diseases (AASLD 2008),
Spanish investigators described findings from the TRIC-1 trial, which assessed
whether adding the insulin-sensitizing drug metformin (Glucopahge) to pegylated
interferon plus ribavirin could improve sustained
virological response (SVR) rates in individuals with insulin resistance.  This
prospective, multicenter, double-blind trial included 125 genotype 1 chronic hepatitis
C patients with baseline insulin resistance (HOMA-IR score > 2) who were randomly
assigned to receive either metformin (425 thrice-daily for the first month, then
850 mg thrice-daily from week 4 to 48) or placebo, in combination with 180
mcg/week pegylated interferon alfa-2a (Pegasys) plus 1000-1200 mg/day weight-adjusted
ribavirin for 48 weeks. Participants who did not achieve early virological
response (EVR) at week 12 or who still had detectable HCV RNA at week 24 discontinued
treatment early. This
prospective, multicenter, double-blind trial included 125 genotype 1 chronic hepatitis
C patients with baseline insulin resistance (HOMA-IR score > 2) who were randomly
assigned to receive either metformin (425 thrice-daily for the first month, then
850 mg thrice-daily from week 4 to 48) or placebo, in combination with 180
mcg/week pegylated interferon alfa-2a (Pegasys) plus 1000-1200 mg/day weight-adjusted
ribavirin for 48 weeks. Participants who did not achieve early virological
response (EVR) at week 12 or who still had detectable HCV RNA at week 24 discontinued
treatment early.
Baseline
characteristics were similar in the 2 arms, including demographic factors, HOMA-IR
scores (about 4.4), and baseline HCV RNA (about 6.4 log10 IU/mL). 57% were men
and the mean age was about 48 years. Results
•
Patients in the metformin arm experienced a greater decrease in HOMA-IT scores
compared with the placebo arm.
•
In an intent-to-treat analysis, overall rates of virological clearance in the
2 arms were as follows:
•
Week 12 (EVR): 54.2% in the metformin arm vs 48.4% in the placebo arm;
•
Week 24: 74.6% vs 75.0%, respectively;
•
Week 72 (SVR): 52.5% vs 42.2%, respectively.
•
Among the 54 women, however, rates of viral clearance were higher in the metformin
arm:
•
Week 12: 57.7% in the metformin arm vs 39.3% in the placebo arm;
•
Week 24: 80.8% vs 71.4%, respectively;
•
Week 72 (SVR): 57.7% vs 28.6%, respectively.
•
Among patients receiving metformin, HCV viral load decreased during the first
12 weeks in a gender-dependent manner.
•
The beneficial effect of metformin was most pronounced in heavier weight women
(> 75 kg, or about 165 lb).
•
Metformin triple therapy was generally well tolerated, but gastrointestinal symptoms
-- mainly mild diarrhea -- were more frequent in the metformin arm.
"Triple
therapy with metformin, peginterferon, and ribavirin was well tolerated, decreased
insulin resistance and increased SVR in this difficult-to-treat group of patients,"
the researchers concluded. "This
therapy was especially effective in females, in whom metformin raised significantly
the SVR rate," they added. "Our data suggest that triple therapy should
be the standard of care for females with hepatitis C genotype 1 and insulin resistance." Pioglitazone  In
a related, but smaller and shorter, study also presented at the conference, another
research team reported results from an analysis of early viral decay in non-diabetic
genotype 1 hepatitis C patients who added pioglitazone (Actos) to pegylated interferon
plus ribavirin. Pioglitazone is a thiazolidinedione anti-diabetes medication from
a different drug class than metformin. In
a related, but smaller and shorter, study also presented at the conference, another
research team reported results from an analysis of early viral decay in non-diabetic
genotype 1 hepatitis C patients who added pioglitazone (Actos) to pegylated interferon
plus ribavirin. Pioglitazone is a thiazolidinedione anti-diabetes medication from
a different drug class than metformin.
In
this study, a group of 10 obese patients (body mass index > 30) received 30
mg/day pioglitazone starting 4 weeks prior to interferon-based therapy and continued
with 4 weeks of combination therapy (8 total weeks of pioglitazone). These patients
were compared with obese and normal-weight control patients (10 each) who received
standard hepatitis C treatment without pioglitazone. Results •
Addition of pioglitazone improved the viral decay rate during the first 4 weeks
of interferon-based therapy.
•
At days 2, 7, 14, and 28, the average decrease in HCV RNA was smaller among obese
patients receiving standard pegylated interferon/ribavirin compared with the lean
participants taking the same regimen (3.8 vs 4.3 log on day 28).
•
However, obese patients who added pioglitazone had a greater decline at each time
point than obese patients on standard therapy (5.3 vs 3.8 log on day 28).
•
At week 4, 60% of obese patients receiving pioglitazone triple therapy achieved
rapid virological response, compared with 50% of normal-weight patients on standard
therapy and 20% of obese patients not taking pioglitazone.
Based
on these findings, the researchers concluded, "Pioglitazone given [as an]
adjuvant to [pegylated interferon/ribavirin] in HCV
genotype 1 patients improves viral kinetic response during the first 4 weeks
of therapy."
11/21/08
References
M Romero-Gomez,
M Diago, RJ Andrade, and others. Metformin with Peginterferon Alfa-2a and Ribavirin
in the Treatment of Naive Genotype 1 Chronic Hepatitis C Patients with Insulin
Resistance (TRIC-1): Final Results of a Randomized and Double-Blinded Trial. 59th
Annual Meeting of the American Association for the Study of Liver Diseases (AASLD
2008). San Francisco. October 31-November 4, 2008. Abstract LB6.
HM Elgouhari,
KB Cesario, R Lopez, and other. Pioglitazone improves early virologic kinetic
response to Peg IFN/RBV combination therapy in hepatitis C genotype 1 naïve
patients. 59th Annual Meeting of the American Association for the Study of Liver
Diseases (AASLD 2008). San Francisco. October 31-November 4, 2008. Abstract 167. | 
![]()
 Several
prior studies have shown that people with
Several
prior studies have shown that people with  This
prospective, multicenter, double-blind trial included 125 genotype 1 chronic hepatitis
C patients with baseline insulin resistance (HOMA-IR score > 2) who were randomly
assigned to receive either metformin (425 thrice-daily for the first month, then
850 mg thrice-daily from week 4 to 48) or placebo, in combination with
This
prospective, multicenter, double-blind trial included 125 genotype 1 chronic hepatitis
C patients with baseline insulin resistance (HOMA-IR score > 2) who were randomly
assigned to receive either metformin (425 thrice-daily for the first month, then
850 mg thrice-daily from week 4 to 48) or placebo, in combination with  In
a related, but smaller and shorter, study also presented at the conference, another
research team reported results from an analysis of early viral decay in non-diabetic
genotype 1 hepatitis C patients who added pioglitazone (Actos) to pegylated interferon
plus ribavirin. Pioglitazone is a thiazolidinedione anti-diabetes medication from
a different drug class than metformin.
In
a related, but smaller and shorter, study also presented at the conference, another
research team reported results from an analysis of early viral decay in non-diabetic
genotype 1 hepatitis C patients who added pioglitazone (Actos) to pegylated interferon
plus ribavirin. Pioglitazone is a thiazolidinedione anti-diabetes medication from
a different drug class than metformin.