| Effectiveness
of Pegylated Interferon plus Ribavirin for Chronic Hepatitis C Patients with HCV
Genotypes 5 or 6 By
Liz Highleyman | Hepatitis
C virus (HCV) genotypes 5 and 6 respond better to interferon-based therapy than
genotype 1, but do not appear as easy to treat as genotypes 2 or 3, according
to 2 studies presented at the recent Disease Week annual meeting (DDW 2009) in
Chicago. |
Introduction Current
standard therapy for chronic hepatitis C is
a combination of pegylated interferon
(Pegasys or PegIntron) plus ribavirin. HCV genotype 1 -- which is most common
in the U.S. -- is the most difficult to treat, with sustained
virological response (SVR) rates around 50% with 48 weeks of therapy; genotype
4 is thought to be similar to genotype 1. Genotypes 2 and 3 respond better, with
SVR rates reaching 70%-80% or higher with 24 weeks of therapy. But
HCV genotype 5, which is seen most often in parts of Africa, and genotype 6, which
predominates in Southeast Asia, are rare in the U.S. and Europe, and their treatment
has not been as extensively studied. Worldwide
distribution of HCV genotypes 1 to 6. | 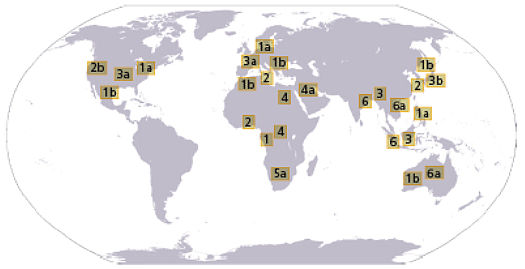 | Source:
Clinical Gastroenterol Hepatol 3: S97. |
Genotypes
5 and 6 in Germany  In
the first presentation, Dietrich Hueppe and colleagues assessed demographic characteristics,
transmission risk factors, and medical care for genotype 5 and 6 chronic hepatitis
C patients in Germany, based on a nationwide non-interventional study conducted
by the Association of German Gastroenterologists in Private Practice in cooperation
with Roche. In
the first presentation, Dietrich Hueppe and colleagues assessed demographic characteristics,
transmission risk factors, and medical care for genotype 5 and 6 chronic hepatitis
C patients in Germany, based on a nationwide non-interventional study conducted
by the Association of German Gastroenterologists in Private Practice in cooperation
with Roche.
From
March 2003 through September 2008, a total of 19,153 patients were included in
the cohort, and 7266 treatments were recorded. The analysis presented at DDW looked
at data from 32 individuals with genotype 5 and 29 with genotype 6. As a proportion
of all hepatitis C cases, the prevalence of these genotypes was very low at 0.17%
and 0.15%, respectively. With
regard to patient characteristics, genotype 5 patients were slightly older than
those with genotype 6 (50 vs 45 years, respectively). Most genotype 6 patients
(79%) were men -- and in most cohorts, men make up a majority of genotype 1, 2,
and 3 patients as well -- but genotype 5 patients were more likely to be women
(59%). Looking
at transmission routes, 56% of genotype 5 patients and 10% of those with genotype
6 cited blood transfusion as a risk factor, while 3% and 10%, respectively, cited
other medical procedures. 13% of genotype 5 and 17% of genotype 6 patients reported
injection drug use, and 3% and 17%, respectively, cited sexual transmission risk
factors. For 28% of genotype 5 and 38% of genotype 6 patients, the transmission
route was unknown. Approximately
60% of these individuals had various comorbidities. Cardiovascular disease, thyroid
disease, diabetes, and thrombocytopenia were predominant among genotype 5 patients,
while hepatitis B virus and HIV coinfection were most frequent among those with
genotype 6. Within
this cohort, 17 individuals with each genotype were treated with pegylated interferon
plus ribavirin. The mean duration of therapy for both genotypes was 41 weeks;
69% of genotype 5 patients and 41% with genotype 6 received at least 80% of the
cumulative prescribed doses of the 2 drugs for 48 weeks. Two patients (12%) with
each genotype discontinued therapy prematurely.
A majority of patients
(75% with genotype 5; 50% with genotype 6) achieved rapid virological response
(RVR), or undetectable HCV RNA at treatment week 4. Almost all (94% and 100%,
respectively) achieved early virological response (EVR) at week 12, but some experienced
viral rebound, so the end-of-treatment (EOT) response rate was 82% for both genotypes.
Some additional patients relapsed after completing therapy, resulting in SVR rates
of 65% for genotype 5 and 59% for genotype 6. "Genotype
5 and genotype 6 patients differed in their baseline demographic and virological
characteristics," the investigators concluded. "However SVR rates between
both genotypes were comparable and seem to be superior to [those of] genotype
1 patients." Center
of Gastroenterology, Herne, Germany; Center of Gastroenterology, Dortmund, Germany;
Center for HIV and Hepatogastroenterology, Duesseldorf, Germany; Center for Liver
and Infectious Diseases, Stuttgart, Germany; Center of Gastroenterology, Goettingen,
Germany; Hospital of Johannes-Gutenberg-Universitaet, Mainz, Germany; Center of
Gastroenterology, Hannover, Germany; Center of Gastroenterology, Kassel, Germany;
Center of Gastroenterology, Augsburg, Germany; Center of Gastroenterology, Paderborn,
Germany; Center of Gastroenterology and Liver Center, Berlin, Germany; Meddata
GmbH, Schkeuditz, Germany; Center of Gastroenterology, Berlin, Germany; Justus-Liebig-Universitaet,
Giessen, Germany; Hepatitis/HIV/CF, Roche Pharma AG, Grenzach-Wyhlen, Germany. Genotype
6 in Viet Nam  In
the second study, Thuy Pham and Dat Ho looked at treatment response among individuals
with HCV genotype 6 in Viet Nam. In
the second study, Thuy Pham and Dat Ho looked at treatment response among individuals
with HCV genotype 6 in Viet Nam.
The
analysis included 75 patients, 42 of them treatment-naive and 33 who had received
previous unsuccessful therapy with conventional interferon. All participants were
treated with 180 mcg/week pegylated interferon alfa-2a (Pegasys) plus 15 mg/kg/day
weight-adjusted ribavirin for 48 weeks. SVR
rates were 69% for treatment-naive patients and 61% for the treatment-experienced
group. At 72 weeks, 74% and 64%, respectively, achieved ALT and AST normalization.
Younger age and AST/ALT ratio < 1 were the factors that predicted sustained
response. Baseline HCV RNA only influenced sustained response in patients with
previous treatment failure. Most people who experienced RVR went on to achieve
SVR. Based
on these findings, the researchers concluded, "Patients with chronic hepatitis
C genotype 6 who have never been treated or have failed with standard interferon
showed good responses when treated by peginterferon alfa-2a combined with ribavirin." "The
sustained viral response was better than that of genotype 1," they continued,
recommending that further studies be done to determine whether treatment duration
might be shortened for genotype 6 patients. Hepatology,
Medic Medical Center, Ho Chi Minh, Viet Nam. 6/19/09 Reference D
Hueppe, E Zehnter, S Mauss, and others. Efficacy and Tolerability of Peginterferon
Alfa-2a (40KD) (PEG) and Ribavirin (RBV) in Genotype 5 and 6 Patients with Chronic
Hepatitis C Under Real Life Conditions. Digestive Disease Week (DDW 2009). Chicago.
May 30-June 4, 2009. Abstract M1794. TT
Phamand DT Ho. Pegylated Interferon Alfa-2a Plus Ribavirin in Chronic Hepatitis
C Patients with Genotype 6. Digestive Disease Week (DDW 2009). Chicago. May 30-June
4, 2009. Abstract M1795.
|