Researchers
Report Promising Results from Studies of 3 Therapeutic HCV Vaccines By
Liz Highleyman
Current
standard therapy for chronic hepatitis C virus (HCV)
infection consists of pegylated
interferon plus ribavirin, and several directly targeted oral
anti-HCV agents are in advanced stages of development. But another approach
-- therapeutic vaccines -- is also under study, as reported in 3 presentations
at the 44th Annual Meeting of the European Association
for the Study of the Liver (EASL 2009) last month in Copenhagen. Therapeutic
vaccines are administered to people who are already infected in an attempt to
reduce viral replication and slow or halt disease progression. This strategy is
distinct from preventive or prophylactic vaccination -- also
under study -- which is intended to prevent initial infection.
ChronVac-C
M.
Matti Sallberg from the Karolinska Institute in Sweden presented findings from
the first clinical trial of a "naked" DNA vaccine delivered via electroporation,
a procedure in which cell permeability is increased by applying electricity. 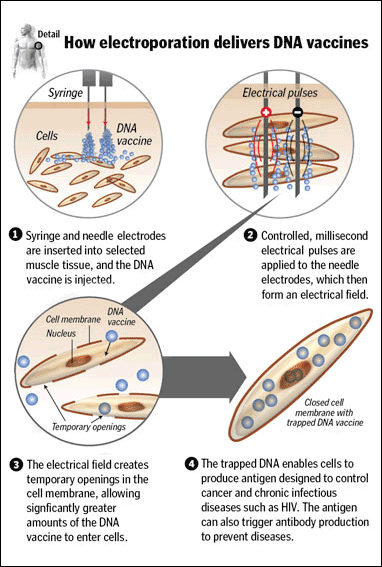
This
ongoing Phase 1/2a trial analyzed the safety and efficacy of ChronVac-C (being
developed by Tripep AB), a T-cell vaccine based on a codon-optimized HCV non-structural
(NS) 3/4A DNA gene expressed under the control of the cytomegalovirus (CMV) immediate-early
promoter.
The study included 12 participants with hard-to-treat HCV
genotype 1, HCV viral load < 800,000 IU/mL, and no previous hepatitis C
treatment. Participants received 0.5 mL subcutaneous injections of saline containing
ChronVac-C DNA, followed by 2 electrical pulses administered via an electrode
array. Patients were divided into groups receiving 167 mcg, 500 mcg, and 1500
mcg of vaccine, given in 4 monthly doses.
Results
 No severe side effects were observed in any of the 3 vaccine dose groups.
No severe side effects were observed in any of the 3 vaccine dose groups.
 In the 167 mcg group, 2 patients mounted transient T-cell responses, but none
had reduced HCV viral load.
In the 167 mcg group, 2 patients mounted transient T-cell responses, but none
had reduced HCV viral load.
 In the 500 mcg group, 2 participants mounted stronger and more sustained HCV-specific
T-cell responses, and both experienced HCV RNA reductions of up to 0.89 and 1.5
log10.
In the 500 mcg group, 2 participants mounted stronger and more sustained HCV-specific
T-cell responses, and both experienced HCV RNA reductions of up to 0.89 and 1.5
log10.
 The third patient in the 500 mcg group had no immune response and no clear reduction
in HCV viral load.
The third patient in the 500 mcg group had no immune response and no clear reduction
in HCV viral load.
 In the 1500 mcg group, 1 patient developed an HCV-specific T-cell response, and
2 experienced viral load reductions of up to 1.2 and 2.4 log10.
In the 1500 mcg group, 1 patient developed an HCV-specific T-cell response, and
2 experienced viral load reductions of up to 1.2 and 2.4 log10.
 Overall, 4 out of 6 patients (67%) in the 2 highest dose groups experienced viral
load reductions exceeding 0.5 log10 and lasting for 2 to > 10 weeks.
Overall, 4 out of 6 patients (67%) in the 2 highest dose groups experienced viral
load reductions exceeding 0.5 log10 and lasting for 2 to > 10 weeks.
 Of these participants, 3 exhibited activation of HCV-specific T-cell responses
at the time of the viral load reductions.
Of these participants, 3 exhibited activation of HCV-specific T-cell responses
at the time of the viral load reductions.
"These
data provide the first proof-of-concept for DNA-based therapeutic vaccination
against chronic hepatitis C in humans using in vivo electroporation and encourage
further clinical development," the researchers concluded. "The data
also provides further evidence for the antiviral role of the HCV-specific T-cell
response."
Karolinska Institutet at Karolinska University Hospital
Huddinge, Stockholm, Sweden; Ludvig-Maximilian University; ImmuSystems GmbH, Munich,
Germany; Inovio Biomedical, Oslo, Norway; Inovio Biomedical, San Diego, CA.
TG4040
In
the second study, F. Habersetzer from Civil Hospital in Strasbourg, France, and
colleagues evaluated Transgene's TG4040 (MVA-HCV), a novel T-cell inducing therapeutic
vaccine based on modified virus Ankara (MVA), a highly attenuated vaccinia poxvirus
strain. This vaccine candidate expresses 3 HCV antigens -- NS3, NS4, and NS5B
-- that are targets of immune responses in individuals who naturally control HCV.
This Phase 1 open-label dose-escalation study evaluated the safety and
biological activity of TG4040 in 15 treatment-naive patients with genotype 1 chronic
HCV infection. Patients in successive cohorts received 3 weekly subcutaneous injections
at escalating doses of 10E6, 10E7, and 10E8 pfu of TG4040 on days 1, 8, and 15.
Participants treated with the highest dose also received a booster shot of the
same dose 6 months after the first injection.
Results
 All studied doses of TG4040 were safe and generally well-tolerated.
All studied doses of TG4040 were safe and generally well-tolerated.
 The most frequent adverse drug reactions were mild-to-moderate injection site
reactions and flu-like illness.
The most frequent adverse drug reactions were mild-to-moderate injection site
reactions and flu-like illness.
 No grade 3/4 or serious drug reactions or adverse events leading to discontinuation
or treatment modification were reported.
No grade 3/4 or serious drug reactions or adverse events leading to discontinuation
or treatment modification were reported.
 6 out of 15 patients experienced decreases in HCV viral load ranging from 0.5
to 1.4 log10 IU/mL from baseline (5 of these participants were enrolled in the
first and second cohorts).
6 out of 15 patients experienced decreases in HCV viral load ranging from 0.5
to 1.4 log10 IU/mL from baseline (5 of these participants were enrolled in the
first and second cohorts).
 In the first 2 cohorts, vaccine-specific interferon-gamma production was detected
in half the participants.
In the first 2 cohorts, vaccine-specific interferon-gamma production was detected
in half the participants.
 The 2 patients who experienced the largest decreases in HCV RNA (0.8 and 1.4 log10)
also exhibited the greatest increases in HCV-specific interferon-gamma-producing
T-cells targeting NS3 and NS4.
The 2 patients who experienced the largest decreases in HCV RNA (0.8 and 1.4 log10)
also exhibited the greatest increases in HCV-specific interferon-gamma-producing
T-cells targeting NS3 and NS4.
 1 of these patients experienced a peak in interferon-gamma-producing cells 1-2
weeks after the third injection, with continued low HCV viral load.
1 of these patients experienced a peak in interferon-gamma-producing cells 1-2
weeks after the third injection, with continued low HCV viral load.
 Most patients in the third cohort had undetectable or low immune responses to
the vaccine and little or no change in HCV RNA levels.
Most patients in the third cohort had undetectable or low immune responses to
the vaccine and little or no change in HCV RNA levels.
These
results "demonstrate that TG4040 is well-tolerated" and provide preliminary
evidence that vaccine-specific immune responses can be mounted in the face of
ongoing viral replication, the investigators concluded, adding, "Such response
can be associated with control of viral load."
In March 2008, the
study was extended to include 27 patients in 3 additional cohorts, in an effort
to fine-tune the timing of the booster dose and evaluate safety in patients with
more advanced liver disease, according to a press release issued by Transgene.
Results from the study extension are expected in the third quarter of 2009, and
the company plans to initiate a Phase 2 trial of TG4040 in combination with pegylated
interferon plus ribavirin starting in early 2010. Another study in Montreal is
evaluating TG4040 in chronic HCV patients who relapsed after prior treatment with
pegylated interferon/ribavirin.
Hepatology and Gastroenterology Department,
Civil Hospital, Strasbourg; Hepatology and Gastroenterology Department, A. Michallon
Hospital, Grenoble; Hepatology and Gastroenterology Department, Hotel-Dieu Hospital,
Lyon; Hepatology and Gastroenterology Department, Brabois Hospital, Nancy; Hepatology
and Gastroenterology Department, Hotel Dieu Hospital, Nantes; Department of Hepatology
and Gastroenterology, Henri Mondor Hospital, Créteil; Transgene, Strasbourg,
France
GI-5005
Finally,
E.J. Lawitz from Alamo Medical Research and colleagues presented findings from
a Phase 2 study of GlobeImmune's GI-5005 or Tarmogen, a vaccine using whole heat-killed
S. cerevisiae expressing HCV NS3 and core antigens.
This open-label
randomized study included 140 genotype
1 chronic hepatitis C patients; 74% were treatment-naive and the rest were
prior non-responders to pegylated
interferon/ribavirin.
Participants were randomized on a 1:1 basis
and stratified according to prior virological response. Arm 1 received a "run-in"
of 5 weekly subcutaneous doses of 40 YU (1 YU = 10,000,000 yeast) GI-5005 monotherapy,
followed by 2 monthly doses of the vaccine, followed by triple therapy consisting
of monthly 40YU GI-5005 injections plus pegylated interferon/ribavirin for 48
weeks. Arm 2 received the standard-of-care regimen without GI-5005.
In
an earlier interim analysis of rapid
virological response (RVR) at week 4, the response rate was more than 2-fold
greater in the GI-5005 triple therapy arm compared with the standard-of-care arm.
The EASL poster presented 12 week data.
Results
 GI-5005 was generally well-tolerated, with no vaccine-related serious adverse
events or dose limiting toxicities.
GI-5005 was generally well-tolerated, with no vaccine-related serious adverse
events or dose limiting toxicities.
 Among participants who could be evaluated for early virological response (EVR;
? 2 log10 drop in HCV RNA at week 12), treatment-naive patients receiving GI-5005
showed a trend toward a higher EVR rate compared with those receiving standard
therapy (94% vs 87%; P = 0.23).
Among participants who could be evaluated for early virological response (EVR;
? 2 log10 drop in HCV RNA at week 12), treatment-naive patients receiving GI-5005
showed a trend toward a higher EVR rate compared with those receiving standard
therapy (94% vs 87%; P = 0.23).
 Among treatment-naive patients with high baseline viral load (> 600,000 IU/mL),
patients receiving GI-5005 triple therapy were also more likely to achieve EVR
than those receiving standard therapy, though the difference again did not reach
statistical significance (93% vs 85%; P = 0.26).
Among treatment-naive patients with high baseline viral load (> 600,000 IU/mL),
patients receiving GI-5005 triple therapy were also more likely to achieve EVR
than those receiving standard therapy, though the difference again did not reach
statistical significance (93% vs 85%; P = 0.26).
 In the small subset of prior non-responders, EVR rates were comparable in the
GI-5005 and standard-of-care arms.
In the small subset of prior non-responders, EVR rates were comparable in the
GI-5005 and standard-of-care arms.
"Triple
therapy with GI-5005 plus [pegylated interferon/ribavirin] was well tolerated
and preliminary data indicate improved EVR rates, despite an unusually high EVR
rate in the [standard-of-care] group," the researchers concluded.
An
additional exploratory analysis at 24 weeks showed a 2-fold improvement in the
proportion of patients with improved serum fibrosis markers (Fibrotest) and a
50% reduction in the number of patients with worsening fibrosis markers in the
GI-5005 triple therapy group. Treatment-naive high viral load patients receiving
triple therapy also had 14% greater likelihood of ALT normalization, according
to a press release issued by GlobeImmune.
Alamo Medical Research, San
Antonio, TX; University of Arizona, Tucson, AZ; University of Colorado Denver,
Aurora, CO; Weill Cornell Medical College, New York, NY; University of Texas Southwestern
Medical Center at Dallas, Dallas, TX; Duke Clinical Research Institute, Duke University
Medical Center, Durham, NC; Scripps Clinical Research Center, La Jolla, CA; Virginia
Commonwealth University Medical Center, Richmond, VA; Baylor College of Medicine,
Houston, TX; Cruickshank and Associates, Santa Barbara, CA; GlobeImmune, Inc.,
Louisville, CO.
5/19/09 References M
Matti Sallberg, L Frelin, H Diepolder, and others. A First Clinical Trial of Therapeutic
Vaccination Using Naked DNA Delivered by In Vivo Electroporation Shows Antiviral
Effects in Patients with Chronic Hepatitis C. 44th Annual Meeting of the European
Association for the Study of the Liver (EASL 2009). Copenhagen, Denmark. April
22-26, 2009. F
Habersetzer, J.-P Zarski, V Leroy, and others. A Novel Vectorized HCV Therapeutic
Vaccine (Tg4040): Results of a Phase I Study in Naive Patients Chronically Infected
by HCV. EASL 2009. Copenhagen, Denmark. April 22-26, 2009. EJ
Lawitz, TD Boyer, GT Everson, and others. GI-5005 Immunotherapy plus Peg-IFN/Ribavirin
versus Peg-Ifn/Ribavirin in Genotype 1 Chronic HCV Subjects; Preliminary Phase
2 EVR Analyses. EASL 2009. Copenhagen, Denmark. April 22-26, 2009.
Other
Sources
European Association for the Study of the Liver. First
Evidence for DNA-Based Vaccination against Chronic Hepatitis C. Press release.
April 24, 2009.
Transgene. Transgene presents additional Phase I data for
TG4040 in hepatitis C chronically infected patients at EASL and is now preparing
for Phase II trial. Press release. April 23, 2009.
GlobImmune. GlobeImmune,
Inc.'s Hepatitis C Therapeutic Vaccine, GI-5005, Improves EVR Rates to 94 Percent
in Phase 2 Clinical Trial. Press release. April 24, 2009. EASL
2009 MAIN PAGE

15th
Conference on Retroviruses and Opportunistic Infections (CROI 2009)
Coverage
by HIV and Hepatitis.com - February 8 - 11, 2009, Montreal
HIV and AIDS Treatment
News, Experimental News, FDA-approved News Highlights
of the 15th Conference on Retroviruses and Opportunistic Infections (CROI 2009)
- Coverage by HIV and Hepatitis.com, February 8 - 11, 2009, Montreal
|
|