Investigational
Drug TBR-652 Demonstrates Dual Activity against CCR5 and CCR2 Co-receptors
 |
 |
 |
 |
 |
 |
 |
| SUMMARY:
Tobira Therapeutics' investigational CCR5 antagonist TBR-652
was shown to have potent anti-HIV activity and it appeared
safe and well-tolerated in its first small trial in people
with HIV, researchers reported last week at the 17th
Conference on Retroviruses & Opportunistic Infections
(CROI 2010) in San Francisco. The drug also blocked CCR2,
suggesting it might be useful as an anti-inflammatory agent.
|
|
 |
 |
 |
 |
 |
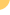 |
 |
By
Liz Highleyman
HIV
can use 2 different surface co-receptors to enter CD4 T-cells, known
as CCR5 and CXCR4. CCR5 antagonists are designed to block one of these
gateways, and people considering such agents require a tropism test
to ensure that they only have virus that uses this co-receptor.
Unlike
the approved CCR5 antagonist maraviroc
(Selzentry) and the more advanced candidate vicriviroc,
TBR-652 also interacts with the CCR2 co-receptor found on monocytes,
dendritic cells, and memory T-cells. CCR2 primarily binds to monocyte
chemoattractant protein 1 (MCP-1). Though not fully understood, CCR2
appears to play a role in inflammation, and it has been linked to inflammatory
diseases including atherosclerosis and metabolic syndrome. To date,
there have been no safety signals associated with blocking this co-receptor.
TBR-652 was previously shown to have promising antiviral activity and
favorable pharmacokinetic properties in laboratory studies and in healthy
HIV negative volunteers. The drug has a half-life of 35-40 hours, making
once-daily dosing feasible. It has good oral bioavailability, but the
primary formulation works better with food. It is neither an inducer
nor inhibitor of CYP450 enzymes, so it is expected to have few drug-drug
interactions
In this Phase 2 proof-of-concept trial, Calvin Cohen from the Community
Research Initiative of New England and colleagues enrolled 54 HIV positive
participants with exclusively CCR5-tropic virus.
Most participants (89%) were men, the average age was about 40 years,
and the mean CD4 cell count was approximately 450 cells/mm3. Participants
were treatment-experienced, but had been off antiretroviral
therapy for at least 6 weeks at study entry and had never taken
any other CCR5 antagonists.
Successive groups of 10 patients were randomly assigned in a 4:1 manner
to receive once-daily TBR-652 monotherapy at escalating does -- 25,
50, 750, 100, or 150 -- or else placebo for 10 days. The 100 mg dose
group used a different formulation than the rest.
Results
 |
By
the end of the 10-day dosing period, HIV viral load had declined
from baseline as follows: |
| |
 |
25
mg group: median 0.5 log drop; |
 |
50
mg group: median 1.3 log drop; |
 |
75
mg group: median 1.6 log drop; |
 |
100
mg group: median 1.2 log drop; |
 |
150
mg group: median 1.5 log drop; |
| Placebo:
0.1 log drop. |
|
 |
Viral
load continued to decrease for up to 4 days after the last dose. |
 |
The
largest median viral load declines (nadirs) in each group were as
follows: |
| |
 |
25
mg group: median 0.7 log drop; |
 |
50
mg group: median 1.7 log drop; |
 |
75
mg group: median 1.8 log drop; |
 |
100
mg group: median 1.4 log drop; |
 |
150
mg group: median 1.7 log drop; |
 |
Placebo:
0.3 log drop. |
|
 |
At
all dose levels, TBR-652 was associated with increases in MCP-1,
with the largest change (about 350-fold) in the 150 mg group, the
smallest (about 5-fold) in the 75 mg group, and essentially none
in the placebo group. |
 |
TBR-652
appeared safe and well-tolerated. |
 |
4
patients in the 25 mg group, 3 in the 50 mg group, none in the 75
mg group, 5 in the 100 mg group, 8 in the 150 mg group, and 3 in
the placebo group reported any adverse events, mostly mild. |
 |
There
were no serious adverse events, laboratory abnormalities, or deaths
during the study. |
 |
The
most common adverse events were gastrointestinal symptoms (e.g.,
nausea, diarrhea, abdominal discomfort) and systemic symptoms (headache,
fatigue, fever). |
 |
There
were no clinically significant changes in heart rhythm. |
 |
No
participants showed evidence of a change from CCR5-tropic to CXCR4-tropic
virus. |
 |
No
TBR-652 resistance mutations were identified in this short study. |
Notably,
TBR-652 did not demonstrate consistent dose relationships. The 2 highest
dose groups experienced the most adverse events without a corresponding
increase in antiviral activity. The 75 mg dose appeared to be optimal
against HIV, with the greatest efficacy (all recipients with >1
log decrease in HIV RNA) and fewest side effects (none); however, it
also had the least CCR2 activity.
Based on these findings, the researchers concluded, "TBR-652 warrants
further investigation as an unboosted, once-daily, oral CCR5 antagonist
with potentially important anti-inflammatory effects."
"These data demonstrate that TBR-652 offers potent viral suppression
and excellent safety and tolerability in this short-term study,"
Cohen stated in a press release issued by Tobira. "This compound
provides the potential for once-daily dosing, without the need for a
pharmacologic boosting agent, an important benefit for simplified dosing
and ease of administration in early stage disease."
"TBR-652's unique properties, including once-daily dosing that
may facilitate co-formulation with other antiretrovirals, such as nucleoside-sparing
or ritonavir-sparing combinations, distinguish it from the early CCR5
antagonists," said Tobira president and CEO James Sapirstein. "Further,
TBR-652's added CCR2 antagonism and potential anti-inflammatory benefits
suggests a bright future for this high-potential compound."
The company indicated that based on the favorable results from this
proof-of-concept trial, it will move forward with Phase 2b studies of
the drug.
Tobira
Therapeutics, Inc, Princeton, NJ; Community Research Initiative of New
England, Boston, MA; AIDS Research Consortium of Atlanta, GA; Orlando
Immunology Ctr, FL; Central Texas Clinical Research, Austin, TX; Whitman
Walker/Elizabeth Taylor Clinic, Washington, DC; CIBIC, Rosario, Argentina.
2/23/10
References
S
Palleja, C Cohen, J Gathe, and others. Efficacy of TBR 652, a CCR5 Antagonist,
in HIV-1-infected, ART-experienced, CCR5 Antagonist-naive Patients.
17th Conference on Retroviruses & Opportunistic Infections (CROI
2010). San Francisco. February 16-19, 2010. Abstract 53.
D Martin,
S Palleja, L Pheng, and others. Pharmacokinetics (PK) and pharmacodynamics
(PD) of TBR-652, a Chemokine Receptor 5 (CCR5) Antagonist, in HIV-infected,
Antiretroviral (ARV) treatment-experienced, CCR5 Antagonist-naive Patients.
17th Conference on Retroviruses & Opportunistic Infections (CROI
2010). San Francisco. February 16-19, 2010. Abstract 598.
Other
source
Tobira Therapeutics. Tobira's Next-Generation Once-Daily CCR5 Receptor
Antagonist Demonstrates Efficacy, Safety and Tolerability in Treatment-Experienced
Patients With HIV. Press release. February 17, 2010 (www.tobiratherapeutics.com).