Daily
Treatment with Valganciclovir Reduces Immune Activation in HIV Positive
People with Cytomegalovirus
 |
 |
 |
 |
 |
 |
 |
| SUMMARY:
Daily treatment with the anti-herpes drug valganciclovir for
8 weeks led to a significant reduction in CD8 T-cell immune
activation in HIV positive people coinfected with cytomegalovirus
(CMV), researchers reported at the 17th Conference on Retroviruses
& Opportunistic Infections (CROI
2010) last month in San Francisco. C-reactive protein
levels appeared to decline, but other inflammation biomarkers
remained stable. |
|
 |
 |
 |
 |
 |
 |
 |
By
Liz Highleyman
Immune activation and inflammation is increasingly recognized as a potential
contributor to non-AIDS conditions in people with HIV, including those
on antiretroviral therapy
(ART) with undetectable HIV viral load. As such, researchers are
exploring the benefits of anti-inflammatory and immune-dampening therapies.
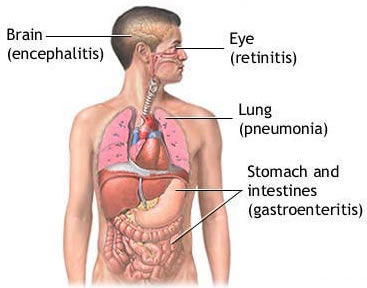 Cytomegalovirus,
a member of the herpesvirus family, is a common latent infection in
both HIV positive and HIV negative people. Responsible for sight-threatening
retinitis in the pre-ART era, it typically does not cause symptomatic
illness as long as immune function remains relatively intact. But CMV
does trigger systemic immune activation, characterized by CD8 T-cells
bearing the CD38 and HLA-DR markers.
Cytomegalovirus,
a member of the herpesvirus family, is a common latent infection in
both HIV positive and HIV negative people. Responsible for sight-threatening
retinitis in the pre-ART era, it typically does not cause symptomatic
illness as long as immune function remains relatively intact. But CMV
does trigger systemic immune activation, characterized by CD8 T-cells
bearing the CD38 and HLA-DR markers.
Peter
Hunt from the University of California at San Francisco and colleagues
previously reported that people with HIV often have a high percentage
of chronically activated CD8 T-cells, and that immune activation is
associated with poor CD4 cell recovery after starting ART.
In the
study presented at CROI, the researchers hypothesized that treating
asymptomatic CMV coinfection might decrease T-cell activation in this
setting, potentially providing an immunologic benefit.
This small pilot study included 30 asymptomatic HIV and CMV positive
participants with a high percentage of CD38 HLA-DR CD8 T-cells. They
had been on stable combination ART for more than 1 year with suboptimal
CD4 cell recovery (< 350 cells/mm3). Most (93%) were men, the median
age was 49 years, 70% had HIV viral load < 75 copies/mL, but the
median CD4 count was just 190 cells/mm3.
Participants were randomly assigned (1:1) to receive either 900 mg/day
valganciclovir or placebo for 8 weeks, followed by a 4-week washout
period. Changes in percentage of activated T-cells, CMV shedding, and
inflammatory biomarkers were assessed every 4 weeks.
Results
 |
At
baseline, 40% of participants had detectable CMV DNA in their saliva,
plasma, or semen. |
 |
None
of the patients receiving valganciclovir showed detectable CMV DNA
at weeks 4, 8, or 12, compared with 44% of placebo recipients. |
 |
At
baseline, the median percentage of activated CD8 T-cells was 20%
in both arms. |
 |
In
the valganciclovir arm, CD8 cell activation decreased by 4.1% at
week 12, a relative reduction of 20%. |
 |
CD8
cell activation remained below the baseline level after the 4-week
washout period in the valganciclovir group. |
 |
Compared
with participants in the placebo arm, those receiving valganciclovir
showed significantly greater reductions in CD8 cell activation at
weeks 8 and 12. |
 |
These
differences remained significant when restricting the analysis to
patients with undetectable plasma HIV RNA. |
 |
High-sensitivity
C-reactive protein (CRP) plasma levels appears to decline in the
valganciclovir arm by week 8, while remaining stable in the placebo
arm. |
 |
However,
plasma levels of other inflammatory, cardiovascular, and kidney
function biomarkers including interleukin 6 (IL-6), D-dimer, and
cystatin C did not differ between the 2 groups. |
 |
There
were also no differences in CD4 T-cell count, percentage of activated
CD4 T-cells, or plasma HIV viral load. |
 |
Valganciclovir
was well-tolerated overall, with no evidence of treatment-related
toxicity including anemia, neutropenia, or kidney dysfunction. |
These
findings led the researchers to conclude, "Valganciclovir durably
suppresses CMV replication and CD8+ T-cell activation in HIV-infected
patients with poor CD4 recovery during ART."
"This
reduction in CD8 activation does not appear to be mediated by a direct
effect on HIV replication, but appears to be the result of reductions
in CMV (or other herpesvirus) replication," they continued. "Thus,
CMV (and possibly other herpesviruses) appears to be a major determinant
of CD8+ T-cell activation during antiretroviral therapy."
"Given
the potential impact of inflammation and immune activation on clinical
outcomes, and the potential role of CMV in cardiovascular disease, T-cell
senescence, and aging, strategies to reduce CMV replication in HIV-infected
individuals are worth pursuing in larger trials," they recommended.
Univ
of California, San Francisco, CA; Univ of Vermont, Colchester, VT; Univ
of Washington, Seattle, WA.
3/5/10
Reference
P
Hunt, J Martin, E Sinclair, and others. Valganciclovir Reduces CD8+
T Cell Activation among HIV-infected Patients with Suboptimal CD4+ T
Cell Recovery during ART. 17th Conference on Retroviruses & Opportunistic
Infections (CROI 2010). San Francisco. February 16-19, 2010. (Abstract
380).