Telaprevir
Improves HCV Cure Rates Regardless of IL28B Status
| SUMMARY:
The Vertex experimental protease inhibitor telaprevir,
taken with pegylated interferon plus ribavirin, increased
sustained response in people with all IL28B gene patterns,
researchers reported at EASL 2011. |
It
is now well-established that variations in the human IL28B gene,
which plays a role in interferon signaling, is a major factor
in determining how well people will respond to interferon-based
therapy for hepatitis C virus
(HCV) infection.
Each
individual inherits 2 copies of each gene, 1 from both parents.
The particular location -- known as a single nucleotide polymorphism,
or SNP -- associated with interferon response can have 3 patterns:
CC (which predicts the best response), CT, and TT.
A
growing body of research is exploring factors associated with
IL28B, including response to new direct-acting antiviral agents.
At the European Association for the Study of the Liver's International
Liver Congress (EASL 2011) last week
in Berlin, investigators with the ADVANCE
and REALIZE trials reported on associations between IL28B
patterns and sustained response to the experimental HCV protease
inhibitor telaprevir.
Below
is an edited excerpt from a press release issued by Vertex describing
these retrospective genetic analyses and their findings.
Data
From Phase 3 Studies Showed Substantial Improvements in SVR
(Viral Cure) Rates With Telaprevir-Based Therapy Compared to
Currently Available Medicines in People With Hepatitis C, Regardless
of Their IL28B Genotype Status
 |
90%
of people with the 'CC' variation of IL28B who were new
to treatment and received a telaprevir-based regimen achieved
a viral cure, 78% of them were eligible to stop all treatment
at 24 weeks. |
 |
Nearly
three-fold improvement in viral cure rates was observed
among people with the 'CT' and 'TT' variations compared
to the control group, regardless of prior treatment experience.
|
Berlin
-- March 31, 2011 -- Vertex Pharmaceuticals Incorporated (Nasdaq:
VRTX) today announced new data from retrospective analyses that
evaluated the relationship between variations at the IL28B gene
and a patient's response to treatment with telaprevir in combination
with pegylated interferon and ribavirin from two of its pivotal
Phase 3 studies (ADVANCE and REALIZE) for a group of people
who were tested for IL28B genotype.
These analyses showed that people in these studies had substantial
improvements in sustained viral response (SVR, or viral cure)
rates across all IL28B genotypes (CC, CT or TT) for those treated
with telaprevir-based combination therapy compared to those
treated with pegylated interferon and ribavirin alone.
Telaprevir is a medicine in development for the treatment of
genotype 1 chronic hepatitis C. Safety and tolerability results
were consistent across the Phase 3 studies of telaprevir. Data
from these IL28B analyses were presented today at The International
Liver Congress 2011, the 46th annual meeting of the European
Association for the Study of the Liver (EASL) in Berlin, Germany.
A specific genetic region near the IL28B gene is referred to
as an IL28B genotype. The three variations of IL28B genotypes
have been associated with a person's response to hepatitis C
treatment with pegylated interferon and ribavirin. The CC variation
is associated with better responses to these medicines.
"Doctors sometimes use IL28B genotype status to decide
which patients should be treated with currently available medicines
because people with the CT and TT variations of IL28B tend to
have substantially lower viral cure rates compared to those
with the CC variation," said Ira Jacobson, MD, Chief of
the Division of Gastroenterology and Hepatology at New York-Presbyterian
Hospital/Weill Cornell Medical Center, and the Vincent Astor
Distinguished Professor of Medicine at Weill Cornell Medical
College and principal investigator for the ADVANCE study. "In
this study, telaprevir was associated with a substantial improvement
over currently available medicines, regardless of IL28B status,
and the greatest improvement was observed in patients with the
CT and TT variations."
In ADVANCE, patients were randomized 1:1:1 to receive telaprevir
(eight weeks or 12 weeks) in combination with pegylated interferon
and ribavirin, followed by pegylated interferon and ribavirin
alone for a total of either 24 weeks or 48 weeks of treatment.
Eligibility for the shorter treatment duration was based on
having undetectable hepatitis C virus at weeks four and 12.
Among patients in this study tested for their IL28B genotype,
90 percent (45/50) of CC patients who received a 12-week telaprevir-based
combination regimen achieved a viral cure and 78 percent (39/50)
of them were eligible to stop all treatment at 24 weeks. These
results were compared to 64 percent (35/55) of patients who
achieved a viral cure with pegylated-interferon and ribavirin
alone for 48 weeks.
"The 90 percent viral cure rate among people with the CC
variation of IL28B in this study is significant, but the fact
that nearly 80 percent of them were eligible for the shorter
course of treatment is an equally important finding," said
Robert Kauffman, MD, PhD, Senior Vice President and Chief Medical
Officer for Vertex. "Vertex plans to conduct a study evaluating
a short-duration, 12-week telaprevir-based regimen in people
who have not been treated for hepatitis C who have the CC variation
of IL28B."
Data from the ADVANCE study showed that patients with the CC
variation of IL28B who were new to treatment and received a
telaprevir-based combination regimen had the highest viral cure
rates compared to those with the CT and TT variations. Data
from both ADVANCE and REALIZE showed a nearly three-fold improvement
in viral cure rates among patients with the CT and TT variations
of IL28B who received telaprevir-based combination therapy compared
to those who received pegylated interferon and ribavirin. These
differences were observed among patients who were new to treatment
as well as those whose prior treatment for hepatitis C was unsuccessful.
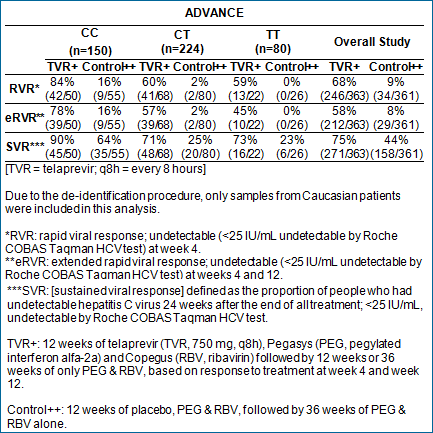
Retrospective Analysis from ADVANCE
The Phase 3 ADVANCE study evaluated people who were new to treatment
for hepatitis C. The retrospective analysis of IL28B status
presented today includes people tested for IL28B genotype (454/1088;
42 percent). Of the patients in ADVANCE who were tested for
their IL28B genotype, the distribution of the variations was
consistent with previously published studies in people new to
treatment. Data from the subanalysis of IL28B status in the
control and telaprevir treatment arms (12 weeks) of the study
are shown in the table.
Retrospective
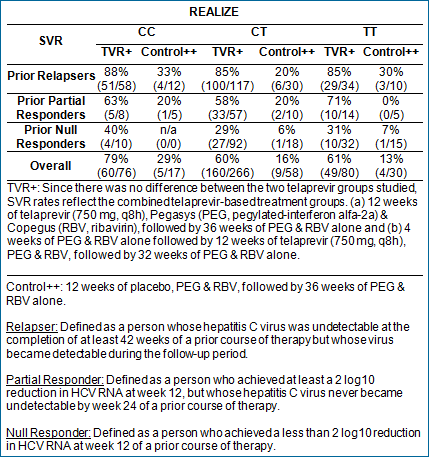
Analysis from REALIZE
The Phase 3 REALIZE study evaluated people whose prior treatment
with pegylated interferon and ribavirin was unsuccessful (prior
relapsers, prior partial responders and prior null responders).
Of the patients in REALIZE who were tested for their IL28B genotype
(527/662; 80 percent), the distribution of patients with the
CT variation was over-represented and the distribution of those
with the CC variation was under-represented. This is consistent
with expectations for a population that has not responded to
a prior course of treatment.
Safety
and Tolerability Information from Phase 3 Studies of Telaprevir
The safety and tolerability results of the telaprevir-based
combination regimens were consistent across the Phase 3 studies.
The most common adverse events were fatigue, pruritus, nausea,
headache, rash, anemia, flu-like symptoms, insomnia and diarrhea
with the majority being mild to moderate. Rash and anemia occurred
more frequently in the telaprevir-based treatment arms compared
to the control groups.
Rash was primarily characterized as eczema-like, manageable
and resolved upon stopping telaprevir. More than 90 percent
of rash was mild to moderate and was primarily managed with
the use of topical corticosteroids and/or antihistamines. Anemia
was primarily managed by reducing the dose of ribavirin.
To optimize each patient's opportunity to achieve viral cure
in the Phase 3 studies, sequential discontinuation of the medicines
was recommended as a strategy to manage certain adverse events.
This strategy allowed patients to continue on pegylated interferon
and ribavirin after stopping telaprevir. Discontinuation of
all medicines due to either rash or anemia during the telaprevir/placebo
treatment phase was 1 percent to 3 percent in the telaprevir
treatment arms.
About IL28B
IL28B is a gene related to the interferon system. A genetic
region near the IL28B gene is referred to as an IL28B genotype.
There are three variations of IL28B genotypes: CC, CT or TT.
These variations have been associated with a person's response
to treatment for hepatitis C with pegylated interferon and ribavirin.
Studies have shown that people with the CC variation respond
better to treatment with pegylated-interferon and ribavirin
than those with the CT or TT variations. The CC variation is
more frequent in Caucasians compared to African Americans (39
percent versus 16 percent), which may partially explain the
lower response to treatment observed among African Americans
in most clinical trials of pegylated-interferon and ribavirin.
About the Phase 3 ADVANCE and REALIZE Studies
ADVANCE was a pivotal Phase 3, randomized, double-blind, placebo-controlled,
global study of 1,088 people who were new to hepatitis C treatment.
The primary endpoint of ADVANCE was SVR (defined as the proportion
of people who had undetectable hepatitis C virus 24 weeks after
the end of all treatment; <25 IU/mL, undetectable by Roche
COBAS Taqman HCV test). The secondary endpoint evaluated the
safety of telaprevir when dosed in combination with pegylated-interferon
and ribavirin.
REALIZE was a pivotal Phase 3, randomized, double-blind, placebo-controlled
study conducted globally with the majority of clinical trial
sites in Europe and North America. The study was designed to
evaluate the efficacy, safety and tolerability of telaprevir-based
combination regimens in people infected with genotype 1 chronic
hepatitis C who did not achieve a viral cure after at least
one course of prior treatment with interferon-based therapy.
Patients were randomized 2:2:1 to two telaprevir-based treatment
arms (simultaneous start and lead-in) and a control arm of pegylated-interferon
and ribavirin alone. The primary endpoint of the REALIZE study
was SVR in each of the two telaprevir treatment arms compared
to the control arm and for the three groups of people included
in the study.
Status of Telaprevir Regulatory Applications
The regulatory applications for the approval of telaprevir have
been granted Priority Review by the U.S. Food and Drug Administration
(FDA) and Health Canada and accelerated assessment by the European
Medicines Agency for the treatment of people with genotype 1
chronic hepatitis C. The FDA has scheduled its Antiviral Drugs
Advisory Committee to discuss the New Drug Application for telaprevir
on April 28, 2011. A target response date of May 23, 2011 is
set under the Prescription Drug User Fee Act (PDUFA). The applications
include data from three registration studies, ADVANCE, ILLUMINATE
and REALIZE, which evaluated telaprevir in combination with
pegylated-interferon and ribavirin in people with hepatitis
C who were new to treatment as well as those who did not achieve
a viral cure after treatment with currently available medicines.
For complete information on the telaprevir clinical trials or
a fact sheet on the trial designs visit: www.vrtx.com/press.cfm.
About the Telaprevir Development Program
Telaprevir is an investigational, oral inhibitor that acts directly
on the HCV protease, an enzyme essential for viral replication.
To date, more than 2,500 people with hepatitis C have received
telaprevir-based therapy as part of Phase 2 studies and the
Phase 3 ADVANCE, ILLUMINATE and REALIZE studies. Together, these
studies enrolled people with genotype 1 chronic hepatitis C
who had not been treated for their disease previously as well
as people who had been treated before but did not achieve a
viral cure.
Vertex is developing telaprevir in collaboration with Tibotec
BVBA and Mitsubishi Tanabe Pharma. Vertex has rights to commercialize
telaprevir in North America. Through its affiliate, Janssen,
Tibotec has rights to commercialize telaprevir in Europe, South
America, Australia, the Middle East and certain other countries.
Mitsubishi Tanabe Pharma has rights to commercialize telaprevir
in Japan and certain Far East countries.
About Vertex
Vertex creates new possibilities in medicine. Our team aims
to discover, develop and commercialize innovative therapies
so people with serious diseases can lead better lives.
Vertex scientists and our collaborators are working on new medicines
to cure or significantly advance the treatment of hepatitis
C, cystic fibrosis, epilepsy and other life-threatening diseases.
Founded more than 20 years ago in Cambridge, MA, we now have
ongoing worldwide research programs and sites in the U.S., U.K.
and Canada.
For more information and to view Vertex's press releases, please
visit www.vrtx.com.
4/5/11
References
IM Jacobson, I Catlett, P Marcellin, et al. Telaprevir substantially
improved SVR rates across all IL28B genotypes in the ADVANCE
trial. 46th Annual Meeting of the European Association for the
Study of the Liver (EASL 2011). Berlin. March 30-April 3. Abstract
7.
S Pol, J Aerssens, S Zeuzem, et al. Similar SVR rates in IL28B
CC, CT or TT prior relapser, partial- or null-responder patients
treated with telaprevir/peginterferon/ribavirin: retrospective
analysis of the REALIZE study. 46th Annual Meeting of the European
Association for the Study of the Liver (EASL 2011). Berlin.
March 30-April 3. Abstract
201.
Other Source
Vertex Pharmaceuticals. Data From Phase 3 Studies Showed Substantial
Improvements in SVR (Viral Cure) Rates With Telaprevir-Based
Therapy Compared to Currently Available Medicines in People
With Hepatitis C, Regardless of Their IL28B Genotype Status.
Press release. March 31, 2011.
