Two Studies Look at Promising Therapies for Liver Cancer By
Liz Highleyman Over
years or decades, a proportion of people with chronic
hepatitis B or C virus infection (HBV, HCV)
will develop hepatocellular
carcinoma (HCC), or primary liver cancer. Liver cancer is the third leading
cause of cancer death worldwide, and in the U.S. rates are rising as people infected
with HCV many years ago reach the later stages of disease.
Unfortunately,
HCC is a difficult malignancy to treat, especially because it is often diagnosed
late. However, recent years have witnessed several advances in treatment, and
survival rates have improved.
Two recently published studies produced promising
data on experimental therapies for HCC: the systemic chemotherapy drug sorafenib
(Nexavar), and combination therapy using doxorubicin-eluting beads plus radiofrequency
ablation. 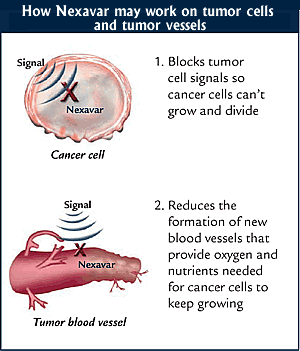
Sorafenib
In
the July 24, 2008 New England Journal of Medicine, investigators reported data
from the pivotal Phase III SHARP (Sorafenib HCC Assessment Randomized Protocol)
trial.
 | Sorafenib
Tablet |
Sorafenib
is an oral multikinase inhibitor of vascular endothelial growth factor receptor,
platelet-derived growth factor receptor, and Raf. Already approved for primary
kidney cancer, the U.S. Food and Drug Administration (FDA) approved sorafenib
in November 2007 for treatment of unresectable (not curable by surgery) HCC, in
part based on the SHARP results.
In this double-blind, multicenter trial,
602 participants with advanced HCC who had not undergone previous systemic treatment
were randomly assigned to receive 400 mg twice-daily sorafenib or placebo. Primary
outcomes were overall survival and time to symptomatic progression. Secondary
outcomes included time to radiologic progression and safety.
Results
•
At the second planned interim analysis, 321 deaths had occurred, and the study
was stopped ahead of schedule.
•
The median overall survival duration was 10.7 months in the sorafenib arm compared
with 7.9 months in the placebo arm (hazard ratio 0.69; P < 0.001).
•
There was no significant difference between the 2 groups in the median time to
symptomatic progression (4.1 vs 4.9 months, respectively; P = 0.77).
•
The median time to radiologic progression was 5.5 months in the sorafenib group
and 2.8 months in the placebo group (P < 0.001).
•
7 patients in the sorafenib group (2%) and 2 in the placebo group (1%) experienced
a partial response.
•
No participants in either group experienced a complete response.
•
Diarrhea, weight loss, hand/foot skin reaction, and hypophosphatemia (elevated
blood phosphate) were more frequent in the sorafenib group.
Based
on these findings, the investigators concluded, "In patients with advanced
hepatocellular carcinoma, median survival and the time to radiologic progression
were nearly 3 months longer for patients treated with sorafenib than for those
given placebo."
As previously reported, researchers presented data
at the 43rd annual meeting of the European Association for the Study of the Liver
(EASL 2008) this past April showing that sorafenib
also extended survival in a subgroup of more than 300 SHARP participants who experienced
failure of prior local HCC therapies including resection (surgery), percutaneous
ethanol injection, radiofrequency ablation, and/or transarterial chemoembolization.
Doxorubicin Beads
+ RF Ablation
Prior
research has shown that a combination
of different HCC therapies can produce better outcomes than single methods
used alone.
As reported in the August 2008 Journal of Hepatology,
Italian researchers assessed the safety and efficacy of doxorubicin-eluting beads
(DEB) - tiny injected spheres that emit a chemotherapy drug - following radiofrequency
(RF) ablation, a method of destroying tumors using heat.
The study included
20 patients with single HCC tumors ranging from 3.3 to 7.0 cm (mean 5.0 cm) who
showed evidence of residual tumor tissue after standard RF ablation. The participants
then underwent intra-arterial DEB administration equivalent to a doxorubicin dose
of 50-125 mg (mean 60.2 mg). The follow-up period ranged from 6 to 20 months (mean
12 months).  | | Radiofrequency
ablation or lesioning is a term used when radio waves are used to produce
heat to destroy tissue, usually a nerve. |
|
Results
•
The volume of treatment-induced necrosis (tissue death) measured by imaging increased
from 48.1 cm3 after RF ablation to 75.5 cm3 after DEB administration.
•
This represented an increase of 60.9%.
•
The enhanced effect resulted in confirmed complete response of the target tumor
in 12 of 20 patients (60%).
•
Incomplete response with persistence of < 10% of initial tumor volume was observed
in 6 patients (30%).
•
Local tumor progression occurred in 2 patients (10%).
•
No major complications occurred, and no deterioration of liver function was observed.
"Intra-arterial
DEB administration substantially enhances the effect of RF ablation," the
study authors concluded. "DEB-enhanced RF ablation is safe and results in
a high rate of complete response in patients refractory to standard RF treatment."
7/25/08
References JM
Llovet, S Ricci, V Mazzaferro, and others. Sorafenib in advanced hepatocellular
carcinoma. New England Journal of Medicine 359(4): 378-390. July 24, 2008.
(Abstract)
LR Roberts.
Sorafenib in liver cancer -- just the beginning. New England Journal of Medicine
359(4): 420-422. July 24, 2008. R
Lencioni, L Crocetti, P Petruzzi, and others. Doxorubicin-eluting bead-enhanced
radiofrequency ablation of hepatocellular carcinoma: A pilot clinical study. Journal
of Hepatology 49(2): 217-222. August 2008. (Abstract)
|
| |
