|
HCV
Genotype 3 May Be Associated with More Rapid Liver Fibrosis
Progression in People with Chronic Hepatitis C
| Infection
with hepatitis C virus (HCV) genotype 3 may increase
the risk of accelerated fibrosis progression compared
with other viral genotypes, according to a Swiss
study published in the October
2009 Journal of Hepatology. This rapid
progression -- combined with good response to interferon
and the fact that experimental oral anti-HCV agents
are less effective against genotype 3 -- suggests
that such patients should receive prompt interferon-based
therapy. |
|
It
is well known that different HCV
genotypes respond differently to interferon-based
therapy for chronic hepatitis
C, with genotypes 2 and 3 considered easiest to treat,
and genotype 1 (and 4 in some studies) yielding the lowest
sustained response rates. It is less clear whether HCV genotype
plays a role in liver
fibrosis severity, however, though genotype 3 has been
linked to steatosis,
or fat accumulation in the liver.
Investigators
with the Swiss Hepatitis C Cohort Study assessed independent
predictors for fibrosis progression among 1189 patients from
the Swiss Hepatitis C Cohort database with at least 1 biopsy
prior to starting interferon-based antiviral treatment and
an assessable date of infection.
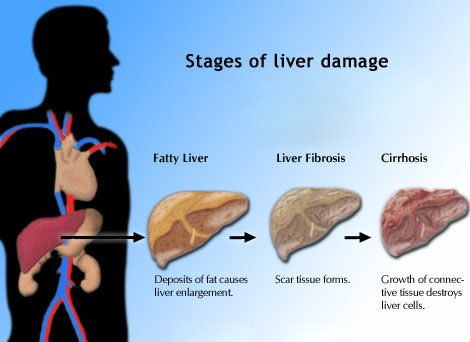
Stage-constant
fibrosis progression rates were assessed using the ratio of
Metavir fibrosis score (F0 through F4) to duration of infection.
Accelerated fibrosis progression was defined as > 0.083
fibrosis units per year.
Results
 |
Independent
risk factors for accelerated stage-constant fibrosis progression
included: |
| |
 |
Male
sex: odds ratio (OR) 1.60 (P < 0.001); |
 |
Older
age at the time of infection: OR 1.08 (P < 0.001); |
 |
Greater
histological activity: OR 2.03 (P < 0.001); |
 |
HCV
genotype 3: OR 1.89 (P < 0.001). |
|
 |
Patients
infected through blood transfusions and invasive medical
procedures or needle sticks had slower fibrosis progression
rates than those infected though sharing equipment for
injection drug use. |
 |
Maximum
likelihood estimates of stage-specific progression rates
(fibrosis units per year) for genotype 3 versus other
genotypes were: |
| |
 |
F0
(absent) to F1 (mild): 0.126 for genotype 2 vs 0.091
for other genotypes; |
 |
F1
to F2 (moderate): 0.099 vs 0.065, respectively; |
 |
F2
to F3 (advanced): 0.077 vs 0.068, respectively; |
 |
F3
to F4 (severe fibrosis or cirrhosis): 0.171 vs 0.112,
respectively. |
|
"This
study shows a significant association of genotype 3 with accelerated
fibrosis using both stage-constant and stage-specific estimates
of fibrosis progression rates," the investigators concluded.
"This observation may have important consequences for
the management of patients infected with this genotype."
"What
are the practical consequences if indeed fibrosis progression
rates are faster in patients infected with HCV genotype 3?"
asked Stefan Zeuzem from JW Goethe University Hospital in
an accompanying editorial.
"First,
comprehensive counseling of patients with respect to proven
(alcohol consumption) or suspected concomitant factors (overweight,
iron overload) is mandatory. Second, reluctance to defer or
delay antiviral therapy may not be appropriate." This
is especially the case given that genotype 3 has a high rate
of sustained virological response to pegylated interferon
plus ribavirin for 24 weeks (70% to 80% in most studies, compared
with about 50% for genotype 1 patients treated for 48 weeks).
While
some people with hepatitis C and their clinicians are awaiting
new oral specifically targeted antiviral therapies (STAT-C),
Zeuzem noted that the drugs furthest along in the development
pipeline -- the HCV protease inhibitors telaprevir
and boceprevir
-- are most active against HCV genotypes 1-2 and less so against
genotypes 3-4, while non-nucleoside HCV polymerase inhibitors
are generally primarily active against genotype 1.
"Taken
together, [the] combination of peginterferon alfa and ribavirin
could remain the key treatment option for patients infected
with HCV genotype 3 in the years to come," Zeuzem concluded.
"If indeed fibrosis progression in patients infected
with HCV genotype 3 is faster than in HCV-1 infected patients,
waiting for new treatment options should be strongly discouraged
in this patient population."
Department
of Internal Medicine, CHUV, Lausanne; Institute of Microbiology,
University of Lausanne, CHUV, Lausanne; Division of Clinical
Pathology, University Hospitals, Geneva; Institute for Social
and Preventive Medicine, CHUV, Lausanne; Division of Clinical
Pharmacology, University Hospital, Bern; Division of Gastroenterology
and Hepatology, University Hospital of Zurich; Division of
Gastroenterology, Canton Hospital, St. Gallen; Division of
Gastroenterology and Hepatology, University Hospital of Basel,
Basel; Division of Gastroenterology and Hepatology, CHUV,
Lausanne; Clinica Moncucco, Lugano,; Pourtalès Hospital,
Neuchâtel; Division of Hospital Preventive Medicine,
CHUV, Lausanne; Division of Gastroenterology and Hepatology,
University Hospitals of Geneva, Geneva, Switzerland.
10/27/09
References
P
Bochud, T Cai, K Overbeck, and others (Swiss Hepatitis C Cohort
Study Group). Genotype 3 is associated with accelerated fibrosis
progression in chronic hepatitis C. Journal of Hepatology
51(4): 655-666. (Abstract).
S
Zeuzem. Forewarned is forearmed. Journal of Hepatology 51(4):
626-627.
(Full
text).
|