Pharmasset
Starts Trial of HCV Polymerase Inhibitor PSI-7977 for Genotype
1 Chronic Hepatitis C Patients
 |
 |
 |
 |
 |
 |
 |
| SUMMARY:
Pharmasset announced last week that it is initiating
a clinical trial to study PSI-7977, a new form of the
nucleotide analog HCV polymerase inhibitor PSI-7851.
The study will evaluate 3 different doses of PSI-7977
in combination with pegylated
interferon plus ribavirin in treatment-naive chronic
hepatitis C patients with hard-to-treat HCV genotype
1, the most common type in the U.S. |
|
 |
 |
 |
 |
 |
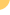 |
 |
Below
is an edited excerpt from a Pharmasset press release describing
the study and the drug.
Pharmasset
Initiates Phase 2a Trial with PSI-7977,
a Chirally Pure Isomer of PSI-7851
Princeton,
NJ -- January 21, 2010 -- Pharmasset, Inc. (Nasdaq: VRUS) today
announces the initiation of a 28-day Phase 2a study with PSI-7977,
a chirally pure isomer form of PSI-7851, a nucleotide analog polymerase
inhibitor in development for the treatment of chronic hepatitis
C (HCV). The trial will evaluate various doses of PSI-7977 in
combination with Pegasys (peginterferon alfa 2a [Pegasys]) and
Copegus (ribavirin) in patients with HCV genotype 1 who have not
been treated previously.
"We
recently reported encouraging clinical results with PSI-7851,"
said Michelle Berrey, MD, MPH, Pharmasset's Chief Medical Officer.
"We now believe there are a number of advantages in moving
forward with a chirally pure isomer of PSI-7851, PSI-7977, including
improvements in manufacturing. We look forward to reporting interim
data from the Phase 2a trial in the third quarter of 2010, and
hope to rapidly progress PSI-7977 into longer-term studies to
demonstrate the safety and efficacy of the nucleosides/tides in
all HCV genotypes."
About PSI-7977
PSI-7851
is a mixture of two molecules of identical chemical composition,
PSI-7976 and PSI-7977, which only differ in the stereo-orientation
of one of the atoms. Once inside a liver cell, both molecules
are rapidly converted to the same active triphosphate. Given the
greater improvements in manufacturing and better in vitro potency,
PSI-7977 was selected for further clinical development. A superior
formulation in the form of a tablet has been developed and has
recently been evaluated in a Phase 1 study in healthy volunteers.
The 28 day Phase 2a trial using the improved manufacturing process
and tablet formulation has now been initiated and interim results
from this study are anticipated in the third quarter of 2010.
The
Phase 2a trial is expected to enroll about 60 patients with chronic
hepatitis C infection who have not been treated previously. The
primary goal of the study is to determine the safety and tolerability
of PSI-7977 in combination with pegylated interferon and ribavirin.
The primary efficacy endpoint of the trial will be the proportion
of patients who achieve a rapid virologic response (RVR), defined
as undetectable levels of HCV (measured by TaqMan assay) four
weeks after the initiation of treatment. Patients will continue
to be followed through a Sustained Virologic Response (SVR) endpoint.
Patients will be randomized to receive one of four treatments:
 |
PSI-7977
100 mg QD [once-daily] in combination with Pegasys and Copegus
for four weeks, followed by 44 weeks of Pegasys and Copegus |
 |
PSI-7977
200 mg QD in combination with Pegasys and Copegus for four
weeks, followed by 44 weeks of Pegasys and Copegus |
 |
PSI-7977
400 mg QD in combination with Pegasys and Copegus for four
weeks, followed by 44 weeks of Pegasys and Copegus |
 |
A
control arm with Pegasys and Copegus |
 Pharmasset
is a clinical-stage pharmaceutical company committed to discovering,
developing, and commercializing novel drugs to treat viral infections.
Pharmasset's primary focus is on the development of oral therapeutics
for the treatment of hepatitis C virus (HCV) and, secondarily,
on the development of Racivir™ for the treatment of human
immunodeficiency virus (HIV). Our research and development efforts
focus on nucleoside/tide analogs, a class of compounds which
act as alternative substrates for the viral polymerase, thus
inhibiting viral replication. We currently have three clinical-stage
product candidates. RG7128, a nucleoside analog for chronic
HCV infection, is in a Phase 2b clinical trial in combination
with Pegasys plus Copegus and is also in the Phase 1b INFORM
studies, the first series of studies designed to assess the
potential of combinations of small molecules without Pegasys
and Copegus to treat chronic HCV. These clinical studies are
being conducted through a strategic collaboration with Roche.
Our other clinical stage candidates include PSI-7977, a chirally
pure isomer of PSI-7851, an unpartnered, next generation HCV
nucleotide analog, that has completed initial Phase 1 clinical
studies which provided supportive safety and efficacy data to
initiate a Phase 2a trial, and Racivir, for the treatment of
HIV, which has completed a Phase 2 clinical trial. We also have
two purine nucleotide analogs, PSI-938 and PSI-879, in advanced
preclinical development. Pharmasset
is a clinical-stage pharmaceutical company committed to discovering,
developing, and commercializing novel drugs to treat viral infections.
Pharmasset's primary focus is on the development of oral therapeutics
for the treatment of hepatitis C virus (HCV) and, secondarily,
on the development of Racivir™ for the treatment of human
immunodeficiency virus (HIV). Our research and development efforts
focus on nucleoside/tide analogs, a class of compounds which
act as alternative substrates for the viral polymerase, thus
inhibiting viral replication. We currently have three clinical-stage
product candidates. RG7128, a nucleoside analog for chronic
HCV infection, is in a Phase 2b clinical trial in combination
with Pegasys plus Copegus and is also in the Phase 1b INFORM
studies, the first series of studies designed to assess the
potential of combinations of small molecules without Pegasys
and Copegus to treat chronic HCV. These clinical studies are
being conducted through a strategic collaboration with Roche.
Our other clinical stage candidates include PSI-7977, a chirally
pure isomer of PSI-7851, an unpartnered, next generation HCV
nucleotide analog, that has completed initial Phase 1 clinical
studies which provided supportive safety and efficacy data to
initiate a Phase 2a trial, and Racivir, for the treatment of
HIV, which has completed a Phase 2 clinical trial. We also have
two purine nucleotide analogs, PSI-938 and PSI-879, in advanced
preclinical development.
For more information, visit http://www.pharmasset.com.
1/29/10
Source
Pharmasset, Inc. Pharmasset Initiates Phase 2a Trial with PSI-7977,
a Chirally Pure Isomer of PSI-7851. Press release. January 21, 2010.
|
