2009
H1N1 Influenza: What Next for People with HIV?
 |
 |
 |
 |
 |
 |
 |
| SUMMARY:
As a new flu season gets underway, a series of recent
reports have looked at aspects of the 2009 H1N1
influenza strain responsible for last year's "swine
flu" epidemic. Spanish researchers reported
the promising finding that people with well-controlled
HIV disease on antiretroviral therapy (ART) had
H1N1 flu outcomes similar to those of HIV negative
individuals. But another pair of studies found that
HIV positive people -- especially those with low
CD4 cell counts -- did not respond as well as HIV
negative people to the H1N1 vaccine or an older
flu vaccine. Finally, scientists with the National
Institute of Allergy and Infectious Diseases (NIAID)
recently projected what might happen with H1N1 this
year and into the future. |
|
 |
 |
 |
 |
 |
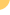 |
 |
The
2009 H1N1 influenza A strain was identified in Mexico in April
2009 and soon reached pandemic levels. While the World Health
Organization (WHO) declared an official
end to the swine flu this past August. That does not mean
this flu strain has gone away. Experts expect that it will
continue to circulate as a seasonal flu variant, though by
now a majority of people are thought to have some degree of
immunity.
H1N1
Outcomes in People with HIV
 In
the October
23, 2010 issue of AIDS, Melchor Riera from Hospital
Son Dureta in Mallorca, Spain, and colleagues described the
clinical presentation and prognosis of HIV positive patients
admitted to hospitals with pandemic H1N1 influenza, as compared
with the general population. In
the October
23, 2010 issue of AIDS, Melchor Riera from Hospital
Son Dureta in Mallorca, Spain, and colleagues described the
clinical presentation and prognosis of HIV positive patients
admitted to hospitals with pandemic H1N1 influenza, as compared
with the general population.
The analysis included 585 adult admitted to 13 hospitals in
Spain with confirmed 2009 H1N1 influenza A between June and
November 2009. Participants were prospectively followed until
1 month after discharge.
Within this population, 26 were HIV positive. The HIV patients
had long-term well-controlled infection. About 90% were on
combination ART (aka HAART), more than 80% had undetectable
HIV RNA, and the median CD4 count was about 500 cells/mm3.
The average age of HIV positive and HIV negative people was
similar (about 40 years), but those in the HIV group were
more likely to be men (69% vs 50%) and less likely to be pregnant
(12% vs 53%). HIV positive people were more often smokers
and were much more likely to have received the H1N1 flu vaccine
(56% vs 11%) and pneumococcal pneumonia vaccine (50% vs 3%).
People with HIV were also more likely to have chronic liver
disease and chronic obstructive pulmonary disease.
The researchers saw no significant differences between HIV
positive and HIV negative people with regard to reported flu
symptoms and physical findings upon hospital admission. About
half of patients in both groups showed abnormal radiological
(x-ray) findings, 30% had respiratory failure, and about 10%
developed secondary pneumonia.
People in both groups started to improve after about 2.5 days
on average and spent a similar amount of time in the hospital
(6-7 days). Almost all patients in both groups received influenza
antiviral therapy and most also received antibiotics. Similar
percentages required intensive care (12%) and mechanical ventilation
(8%-9%). There were no observed differences in clinical outcomes,
and most people recovered; no HIV positive patients and only
3 HIV negative patients died.
"In HIV patients, well controlled on HAART, the pandemic
influenza virus A H1N1 had a similar clinical outcome and
prognosis to that of non-HIV patients," the study authors
concluded.
H1N1
Vaccine
A pair of studies in the September 10, 2010 issue of AIDS
looked at flu vaccine outcomes in people with HIV.
In the first
study, Pablo Tebas and colleagues evaluated the safety
and immunogenicity (immune protection) of the 2009 H1N1 vaccine
in 120 HIV positive adults seen at a University of Pennsylvania
hospital in Philadelphia. About 70% were men, a similar percentage
were black, and the median age was 46 years. In this study,
all participants except 1 were on ART and more than 90% had
undetectable viral load. The median current CD4 cell count
was 502 cells/mm3 and the median nadir (lowest-ever) count
was 132 cells/mm3.
All participants received a single 15 mcg intramuscular dose
of a monovalent, unadjuvanted (that is, not containing extra
ingredients to boost immune response), inactivated H1N1 vaccine.
At baseline, 25% had antibody evidence of previous H1N1 exposure.
Among HIV positive people those without prior exposure, 61%
develop protective antibody titers by week 3 after vaccination,
compared with typical rates of around 90% for HIV negative
people. Non-responders had lower current and nadir CD4 cell
counts and less time with suppressed viral load. Among the
9 patients with detectable viral load, only 4 -- less than
half -- developed protective immunity.
Given this suboptimal response, the researchers recommended,
"Alternative vaccines, dosing, adjuvants, or schedule
strategies are needed to achieve effective immunization of
this vulnerable population."
In a second
study in the same issue, Stefanie Fritz from Basel University
in Switzerland and colleagues conducted a prospective study
of a different flu shot -- the 2007-2008 seasonal flu vaccine
-- in 31 HIV positive and 24 HIV negative people. All HIV
positive participants had been on ART for at least 3 months
and had HIV RNA < 200 copies/mL.
In this analysis, HIV positive people with CD4 cell counts
below 350 cells/mm3 were significantly less likely to develop
protective H1N1-specific T-cell responses than those with
higher counts, who in turn were less likely than HIV negative
individuals (22%, 64%, and 92%, respectively). In the low
CD4 group, H1N1-specific immunoglobulin M (IgM) antibody responses
were absent, and expansion of interferon-gamma-secreting CD4
T-cells was impaired. However, immunoglobulin G (IgG) responses
from memory cells were seen in all 3 groups.
These findings led the investigators to suggest that, "establishing
broad influenza-specific (immunoglobulin G) B-cell memory
prior to severe immunodeficiency is important," and they
recommend that HIV positive people should receive flu vaccines
regardless of CD4 count.
A related Canadian study presented at the 48th Annual Meeting
of the Infectious Diseases Society of America in October found
that children with HIV have suboptimal response even to an
adjuvanted H1N1 vaccine, with only about 30% achieving adequate
protection.
Fate
of H1N1
In the September
28, 2010 issue of the open-access online journal mBio,
Anthony Fauci and colleagues from the National Institute of
Allergy and Infectious Diseases speculated on the future of
H1N1 swine flu, based on the fate of previous pandemic influenza
viruses.
Below
is the text of a NIAID press release describing their analysis.
What
Next for the 2009 H1N1 Influenza Pandemic?
Washington,
DC -- September 28, 2010 -- Now that the H1N1 influenza pandemic
is officially over, what will happen to the virus? In a perspective
article published today in the online open-access journal mBio,
scientists from the National Institutes of Health delve into
history and explore the fates of other pandemic influenza viruses
in order to speculate on the future of the most recent pandemic
virus.
"While human influenza viruses have often surprised us,
available evidence leads to the hope that the current pandemic
virus will continue to cause low or moderate mortality rates
if it does not become extinct," write Anthony Fauci, Director
of the National Institute of Allergy and Infectious Diseases
(NIAID) and his NIAID coauthors, Jeffery Taubenberger and David
Morens.
The impact of the virus in the upcoming influenza season will
depend directly on the degree of existing immunity in the population,
provided the virus does not undergo any changes. The authors
currently estimate that approximately 59% of the United States
population has some level of immunity due to either exposure
to the pandemic H1N1 (pH1N1) virus, vaccination or exposure
to a closely related influenza virus. That number will continue
to increase through immunization with the 2010-2011 seasonal
influenza vaccines, which will contain the pH1N1 strain.
In order to continue to survive in a population with such a
high immunity, the pH1N1 virus must undergo either an abrupt
or a gradual change. In the article, the authors look at the
last six influenza pandemics, going back over 163 years, and
examine how those viruses adapted. While some died out for reasons
not entirely understood, others, like the 1889 and 1918 pandemics,
experienced an explosive recurrence. Explosive recurrence of
pH1N1 is not very likely because of the already high and increasing
population immunity.
"Past history and current understanding suggest cautious
optimism that pH1N1 will eventually adapt to stable circulation
via genetic changes resulting in continuing moderate or low
mortality rates or possibly even disappear entirely," the
NIAID scientists write .
Despite their cautious optimism, the authors warn against complacency.
Other post-pandemic viruses have continued to cause various
rates of excess mortality among younger persons for years after
the pandemic appearance and the bulk of the still susceptible
population spans the under-50 age group. For that reason they
recommend infants older than six months, children, teens and
young adults be aggressively targeted for seasonal influenza
vaccination for not only their own protection, but to increase
the overall population immunity.
Investigator
affiliations:
Riera study: Palma De Mallorca, Hospital Son Dureta, Mallorca,
Spain.
Tebas study: University of Pennsylvania School of Medicine, Philadelphia,
PA.
Fritz study: Department of Biomedicine, Basel University, Basel,
Switzerland.
Brophy study: Departments of Pediatrics and Infectious Diseases,
Children's Hospital of Eastern Ontario, Ottawa, Canada; Infectious
Diseases, McGill University, Montreal, Quebec, Canada; Department
of Pediatrics, Hospital for Sick Children, Toronto, Ontario, Canada.
11/16/10
References
M Riera,
A Payeras, MA Marcos, and others. Clinical presentation and prognosis
of the 2009 H1N1 influenza A infection in HIV-1-infected patients:
a Spanish multicenter study. AIDS 24(16): 2461-2467 (Abstract).
October 23, 2010.
P Tebas, I Frank, M Lewis, and others. Poor immunogenicity of the
H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS
24(14): 2187-2192 (Abstract).
September 10, 2010.
S Fritz, E Mossdorf, B Durovic, and others. Virosomal influenza-vaccine
induced immunity in HIV-infected individuals with high versus low
CD4+ T-cell counts: clues towards a rational vaccination strategy.
AIDS 24(14): 2287-2289 (Abstract).
September 10, 2010.
G Reyes-Teran and ST Butera. Preventing influenza coinfection among
HIV-infected persons: a complex picture coming into focus (Editorial
Comment). AIDS 24(14): 2283-2285.
JC Brophy, B Ward, LM Samson, and others. Immunogenicity of AS03-Adjuvanted
H1N1 Pandemic Influenza Vaccine in HIV-Infected Children. 48th Annual
Meeting of the Infectious Diseases Society of America. Vancouver,
October 21-24, 2010. (Abstract
1509).
DM Morens, JK Taubenberger, AS Fauci. and others. The 2009 H1N1
pandemic influenza virus: What next? mBio 1(4): e00211-10
(free
full text). September 28, 2010.

|
