CDC
Issues Guidance for Truvada Pre-exposure Prophylaxis
 |
 |
 |
 |
| SUMMARY:
The U.S. Centers for Disease Control and Prevention
(CDC) this week issued interim guidance regarding
the use of tenofovir/emtricitabine
(Truvada) for pre-exposure prophylaxis (PrEP)
against HIV infection. The guidelines, published
in the January
28, 2011, Morbidity and Mortality Weekly Report,
note the limitations of the recent iPrEx trial and
recommend PrEP only for gay/bisexual men with "substantial,
ongoing, high risk" for acquiring HIV. |
|
 |
 |
 |
 |
By
Liz Highleyman
 As
reported this past fall, the iPrEX trial, described in
the November 23, 2010, advance online edition of the New
England Journal of Medicine (December 30, 2010 print edition),
was the first to show that daily oral tenofovir/emtricitabine
PrEP could reduce the risk of HIV infection in humans.
As
reported this past fall, the iPrEX trial, described in
the November 23, 2010, advance online edition of the New
England Journal of Medicine (December 30, 2010 print edition),
was the first to show that daily oral tenofovir/emtricitabine
PrEP could reduce the risk of HIV infection in humans.
This
study -- which included more than 2000 men who have sex with
men (MSM) in South America, South Africa, Thailand, and the
U.S. (Boston and San Francisco) -- found that those who used
PrEP daily were 44% less likely to acquire HIV, while the
subset of men who achieved good adherence lowered their risk
by 73%.
The
trial results were greeted with enthusiasm -- especially in
the wake of disappointing results from several previous biomedical
prevention trials -- but also numerous questions about who
could potentially benefit, the long-term risks of tenofovir/emtricitabine,
and cost and access issues.
While
Truvada is not approved by the U.S. Food and Drug Administration
(FDA) for HIV prevention, doctors may prescribe drugs for
off-label use, and some gay men are eager to start using PrEP
right away. The new guidance is intended to offer instructions
and cautions for people interested in using PrEP now, while
awaiting more extensive clinical trial data regarding longer-term
use and other at-risk populations. The CDC and Public Health
Service are currently working on more definitive guidelines.
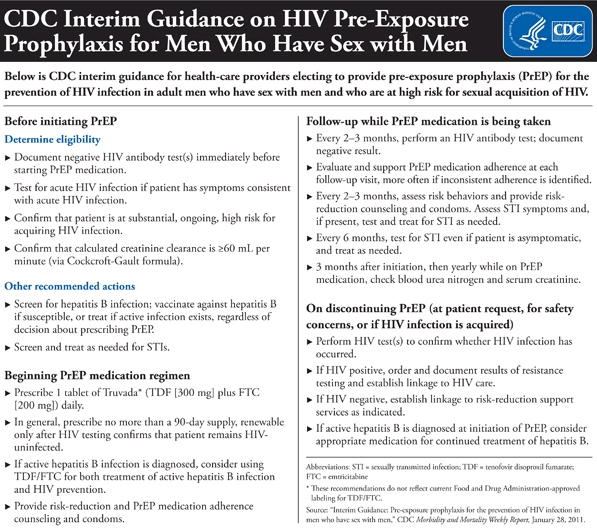
The
CDC has provided a chart summarizing the guidance, included
below and available online at www.cdc.gov/nchhstp/newsroom/PrEPMSMGuidanceGraphic.html.
Key
points include:
Click
Here To View High Resolution Version of Below Graphic
In an editorial note accompanying the MMWR report of
the iPrEx data, the authors elaborated, "When evaluating
MSM for the prescription of PrEP medications, it is important
to establish whether other effective risk-reduction measures
(e.g., condom use) are not being used consistently and to
ascertain that the risk for HIV acquisition is high (e.g.,
frequent partner change or concurrent partners in a geographic
setting with high HIV prevalence) because these patients might
benefit most from the addition of PrEP to their HIV prevention
regimen."
"PrEP has the potential to contribute to effective and
safe HIV prevention for MSM," they continued, "if
1) it is targeted to MSM at high risk for HIV acquisition;
2) it is delivered as part of a comprehensive set of prevention
services, including risk-reduction and PrEP medication adherence
counseling, ready access to condoms, and diagnosis and treatment
of sexually transmitted infections; and 3) it is accompanied
by monitoring of HIV status, side effects, adherence, and
risk behaviors at regular intervals."
Investigator affiliation: National Center for HIV/AIDS,
Viral Hepatitis, STD, and TB Prevention, CDC.
2/1/11
Reference
DK Smith, RM Grant, PJ Weidle, and others (CDC). Interim
Guidance: Preexposure Prophylaxis for the Prevention of HIV
Infection in Men Who Have Sex with Men. Morbidity and Mortality
Weekly Report 60(03): 65-68 (abstract).
January 28, 2011.
Other
Source
CDC
NCHHSTP Media Team. CDC Issues Interim Physician Guidance
on PrEP for MSM. Media advisory. January 27, 2011.
